Abstract
Delay discounting (DD), a person’s preference for smaller immediate rewards over larger delayed rewards, is a heritable trait that is associated with psychiatric and physical outcomes, yet the biological mechanisms underlying these links are not known. We performed a GWAS of DD using 134,935 23andMe research participants and identified 11 genome-wide significant loci. We did not replicate our previously reported association with rs6528024 (chrXq13.3, GPM6B; P = 5.30 × 10−02). The SNP-heritability of DD was 9.85 ± 0.57%. We observed genetic correlations between DD and 73 behavioral, physical, and neuroimaging traits, many of which persisted even after accounting for educational attainment, intelligence, and executive function. Network analysis revealed that the associations between DD and certain traits were explained by both overlapping and trait-specific biological processes. In a hospital-based cohort (N = 66,917), DD polygenic scores were associated with 212 medical conditions. These results demonstrate that DD has a pleiotropic and polygenic common variant architecture, and is genetically associated with numerous outcomes, making it a promising endophenotype for psychiatric and physical health.
Similar content being viewed by others
Introduction
Delay discounting (DD) is the tendency for individuals to devalue delayed rewards [1]. DD belongs to a broader family of traits that measure aspects of cognitive and executive function, including decision-making [2, 3] and impulsivity [4, 5]. In humans, DD is typically measured using a hypothetical monetary temporal discounting task [1] wherein participants make a series of choices between a smaller, immediate reward and a larger, delayed reward. These choices can be modeled as a hyperbolic discounting curve, which may be steeper (i.e., greater discounting of delayed rewards) or shallower. Steeper discounting is positively correlated with substance use disorders (SUDs) [6,7,8], behavioral addictions (e.g., gambling disorder) [9], body mass index (BMI) [10, 11], attention-deficit hyperactivity disorder (ADHD) [12], bipolar disorder [10], schizophrenia [10, 13, 14], and post-traumatic stress disorder (PTSD) [15]. In contrast, shallower DD is observed in obsessive-compulsive disorder, certain personality disorders [16], and anorexia nervosa [10, 17, 18]. Based on this spectrum of associations, DD has been proposed as a transdiagnostic marker of health, yet the mechanisms mediating these associations are poorly defined.
Several lines of evidence indicate that DD is heritable. Twin studies have estimated the heritability of DD to be from 35 to 62% [19, 20]. More recently, our prior genome-wide association study (GWAS; N = 23,217) [21] estimated the SNP heritability of DD to be 12.2%, and showed positive genetic correlations between greater DD and ADHD (rg = 0.37), smoking initiation (rg = 0.32), BMI (rg = 0.18), as well as negative genetic correlations with schizophrenia (rg = −0.22), educational attainment (rg = −0.93) and childhood IQ (rg = −0.63). The present study was designed to further characterize the relationship between DD and an array of psychiatric, physical, and cognitive measures. We built upon our prior DD GWAS [21] by increasing the sample size by more than 5-fold to 134,935 US-based 23andMe, Inc. research participants most genetically similar to European reference panels [22]. We performed global [23, 24] and local [25] genetic correlations and conducted network analyses to identify specific biological processes shared between DD and various cognitive and health-related traits. Considering the strong genetic correlations between DD, educational attainment, and IQ [21], we used multivariate techniques to parse the genetic contributions specific to DD from those shared with other cognitive traits. Finally, to further examine the link between genetic liability for DD and medical outcomes, we calculated DD polygenic scores (PGS) in a hospital-based cohort and conducted a polygenic phenome-wide association study (PheWAS).
Results
GWAS of DD uncovered associations with 11 independent loci
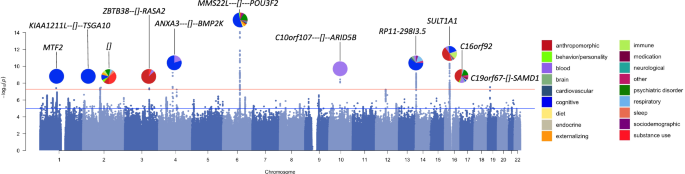
We measured DD in US-based adult research participants (for participant demographics, see Supplementary Table 1) using the 27-item Monetary Choice Questionnaire (MCQ) [1], which was administered via 23andMe’s user portal. DD was summarized using the temporal discounting value (k) [26], wherein a larger value reflects a steeper discounting rate (i.e., a preference for immediate rewards over delayed gratification; see Methods, Supplementary Table 2). As in our prior GWAS, we log10 transformed k to better approximate a normal distribution. Only the participants who clustered within a European genetic ancestry panel (N = 134,935; hereafter referred to as “European cohort”) were used for GWAS (see Supplementary Methods). This cohort includes individuals from our prior publication [21]. We performed a GWAS using an additive genetic model that included age, sex, the first 5 genetic principal components, and indicator variables for genotype platforms as covariates (for single nucleotide polymorphism [SNP] quality control and inclusion, see Supplementary Table 3). Using Linkage Disequilibrium Score Regression (LDSC) [23, 24], we estimated the SNP-based heritability (h2SNP) to be 9.85 ± 0.57% (Intercept: 1.01 ± 0.01). We identified 11 independent loci exceeding the genome-wide significance threshold of P < 5.00 × 10−08 (Fig. 1; Supplementary Tables 4, 5; see Supplementary Figures 1–11 for LocusZoom plots).
Pie plots show categories, highlighted using different colors, previously associated with loci (gene context annotated) from the GWAS catalog [149].
All lead SNPs except for rs57823886 (chr19p13.12) were located within loci that had prior published GWAS associations with at least 1 and up to 60 other traits, most of which were behavioral traits (Fig. 1, Supplementary Table 6). For example, the strongest association (rs34645063, P = 8.60 × 10−15) was located on chr6q16.1 between the genes MMS22L and POU3F2. SNPs in linkage disequilibrium with rs34645063 have previously been implicated in a variety of GWAS including risk-taking behaviors [27, 28], substance use (i.e., alcohol consumption [29], smoking initiation [29,30,31,32], caffeine intake [33, 34]), psychiatric disorders (e.g., bipolar disorder) [35,36,37,38,39], externalizing psychopathology [40], neuroticism/mood instability [41, 42], as well as educational attainment [43, 44], intelligence [45,46,47,48,49], socioeconomic factors (i.e., household income [50]), and BMI [51,52,53]. Notably, we did not replicate our previously reported association with rs6528024 (chrXq13.3, P = 5.30 × 10−02) [21].
Functional annotation identified 93 DD candidate genes
To identify potential candidate genes associated with DD, we performed gene-based (i.e., MAGMA [54], H-MAGMA [55, 56]) and transcriptome-based analyses (i.e., S-PrediXcan [57,58,59], Supplementary Tables 7–10). MAGMA, which maps SNPs to genes based on physical proximity, identified 25 genes. 20 (80%) of these genes were located within 5 of the 11 GWAS loci (Supplementary Table 7). Next, we used H-MAGMA to incorporate GWAS results with chromatin interaction profiles from human brain tissues and iPSC lines, which implicated 66 unique genes across different cell-types (27.84% iPSC derived neurons, 24.74% midbrain dopamine neurons, 24.74% cortical neuron, 22.68% iPSC derived astrocyte) and developmental stages (50.82% fetal brain, 49.18% adult brain; Supplementary Table 8). Finally, we used S-PrediXcan to identify correlations between DD and predicted gene expression in the brain. This analysis identified 33 unique genes, 6 of which were consistently upregulated (i.e., EIF3C, HAUS4, LYG1, NPIPB6, SULT1A2) and 9 of which were consistently downregulated (i.e., CCDC18, INO80E, NPIPB7, NPIPB9, RP11-1348G14.4, SH2B1, SULT1A1) in more than one brain region (Supplementary Table 9). Overall, these analyses identified 93 unique genes associated with DD (Supplementary Table 10), of which 27 (29.03%) were identified by more than one method. Of these genes, 47 (50.54%) were located within or near the 11 loci identified by our GWAS; the most notable of these were the 18 (19.35%) genes within the ch16p11.2 GWAS locus.
DD was globally genetically correlated with 73 psychiatric, cognitive, and physical health traits
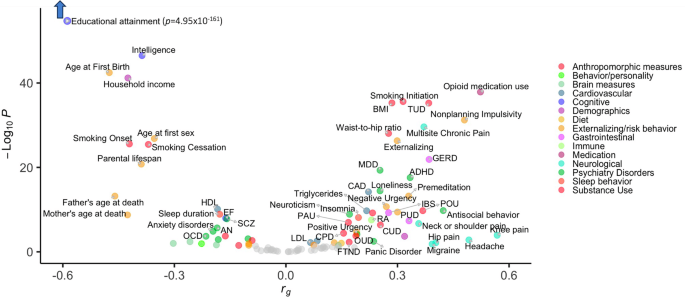
We used LDSC [23, 24] to estimate genome-wide (or “global”) genetic correlations (rg) between DD and 109 other published GWAS traits, which were selected based on previously known phenotypic correlations (e.g., SUDs, impulsivity measures, metabolic traits). DD was genetically correlated with 73 of the 109 traits, including substance use, psychiatric disorders, impulsivity, cognition, physical health, brain measures, and sociodemographic variables (Fig. 2, Supplementary Table 11).
73 FDR-significant results are colored and labeled. See Supplementary Table 11 for complete results.
Substance use and SUDs
DD showed positive genetic correlations with several substance use traits (e.g., smoking initiation rg = 0.32 ± 0.02, problematic alcohol use rg = 0.17 ± 0.03) and SUDs (e.g., tobacco use disorder [TUD] rg = 0.38 ± 0.03, cannabis use disorder rg = 0.25 ± 0.05, and a genetically derived trait capturing shared liability to SUDs, “addiction risk factor” rg = 0.23 ± 0.04).
Psychiatric disorders
DD showed positive genetic correlations with some psychiatric conditions (i.e., antisocial behavior rg = 0.42 ± 0.07, ADHD rg = 0.33 ± 0.04, major depressive disorder rg = 0.25 ± 0.03, loneliness rg = 0.25 ± 0.03, panic disorder rg = 0.24 ± 0.08, suicide attempt rg = 0.19 ± 0.05), but negative genetic correlations with others (e.g., obsessive-compulsive disorder rg = −0.22 ± 0.06, anorexia nervosa rg = −0.19 ± 0.04, autism spectrum disorder rg = −0.18 ± 0.06; schizophrenia rg = −0.16 ± 0.03, bipolar disorder rg = −0.10 ± 0.03).
Impulsivity and externalizing behavior
DD showed positive genetic correlations with some impulsivity facets (e.g., nonplanning impulsivity rg = 0.48 ± 0.04, premeditation rg = 0.33 ± 0.04) and small negative genetic correlations with others (i.e., sensation seeking rg = −0.10 ± 0.04, perseverance rg = −0.10 ± 0.05). We also observed a positive genetic correlation with externalizing (rg= 0.30 ± 0.03).
Cognition and executive function
DD showed negative genetic correlations with cognitive-related traits, including educational attainment (rg = −0.57 ± 0.02), intelligence (rg = −0.39 ± 0.03), and executive function (rg = −0.16 ± 0.03). These traits included the strongest and most significant genetic correlations.
Physical health conditions
DD showed positive genetic correlations with pain-related traits (e.g., migraine rg = 0.39 ± 0.16, multisite chronic pain rg = 0.37 ± 0.03). We observed other positive genetic correlations between DD and digestive (e.g., gastro-esophageal reflux disease rg = 0.39 ± 0.04, irritable bowel syndrome rg = 0.28 ± 0.05), cardiovascular (e.g., coronary artery disease rg = 0.22 ± 0.03), immune (i.e., rheumatoid arthritis rg = 0.23 ± 0.04), sleep (e.g., insomnia rg = 0.20 ± 0.03), and anthropometric variables (e.g., BMI rg = 0.29 ± 0.02, waist-to-hip ratio rg = 0.28 ± 0.03).
Brain measures
DD showed a positive genetic correlation with limbic network structural connectivity (rg = 0.14 ± 0.07). There were negative genetic correlations with functional connectivity of limbic (rg = −0.30 ± 0.12) and somatomotor (rg = −0.19 ± 0.08) networks, and with intracranial volume (rg = −0.26 ± 0.09).
Sociodemographics
DD was negatively genetically correlated with sociodemographic variables, such as household income (rg = −0.43 ± 0.03) and parental lifespan (rg = −0.39 ± 0.04).
Local genetic correlation analysis revealed 3 pleiotropic hotspots, as well as 77 other loci of interest
To more finely pinpoint the specific loci contributing to global genetic correlations, we performed Local Analysis of [co]Variant Annotation (LAVA) [25] across 2,495 semi-independent, approximately equally-sized (~1 Mb) genomic loci, which we downloaded from the LAVA website (https://github.com/cadeleeuw/lava-partitioning). Of the 73 globally genetically correlated traits, 36 traits showed local genetic correlations, which were dispersed across 80 loci (Supplementary Figure 12, Supplementary Table 12). The greatest number of overlapping loci were with intelligence (N = 12 loci).
We detected 3 pleiotropic loci or “hotspots” – chr6q16.1, chr3p21.31, and chr5q14.3 – where DD was locally genetically correlated with 5 or more traits (Supplementary Figure 13). Local versus global genetic correlations were not always consistent. At the chr6q16.1 locus (comprising 14 genes), which was the most pleiotropic hotspot, we observed consistent negative local correlations with educational attainment, intelligence, bipolar disorder, age at first birth, systolic blood pressure, infant head circumference at 6–30 months old, and executive function, all of which were consistent with the global genetic correlations. We also observed an inconsistent negative genetic correlation with externalizing at this locus, which showed a positive global genetic correlation. At the chr3p21.31 locus (127 genes), we identified positive genetic correlations with gastro-esophageal reflux disease, multisite chronic pain, externalizing, major depressive disorder, and ADHD, which were consistent with the global genetic correlations, as well as circumference at birth, which had shown a negative global genetic correlation. Finally, at the chr5q14.3 locus (10 genes), we observed 2 consistent positive genetic correlations with cannabis use disorder and loneliness, 2 consistent negative genetic correlations with executive function and intelligence, and 1 inconsistent positive genetic correlation with infant head circumference, which had shown a negative global genetic correlation.
Global genetic correlations may not reflect the strength of local genetic correlations if only a few loci are genetically correlated. For example, 9 traits showed local genetic correlations but no global genetic correlations with DD. 7 of these traits were locally genetically correlated with DD at only 1 or 2 loci (Supplementary Figure 12, Supplementary Table 12).
Finally, we found that DD was genetically correlated with 10 traits at only one locus per trait (Supplementary Table 12). For example, we found a single positive local genetic correlation between DD and addiction risk factor at the chr11q23.1 locus, which contains the NCAM1-TTC12-ANKK1-DRD2 gene cluster.
Network analysis identifies overlapping and distinct biological processes underlying the relationships between DD and other complex traits
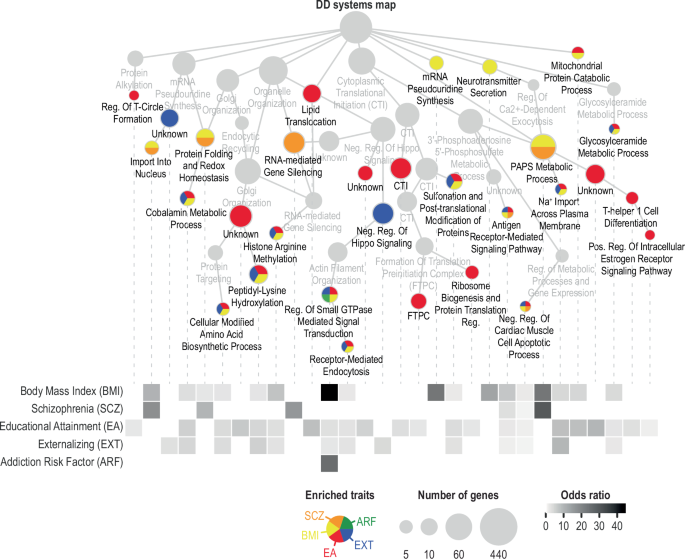
To characterize the biological processes shared between DD and other traits, we propagated MAGMA-identified DD genes in HumanNet-XC [60, 61] (Supplementary Figure 14; Supplementary Table 13). HumanNet-XC is a gene interaction network that integrates gene co-expression, protein-protein interactions, and genetic interactions to model human biology and disease. We selected 6 traits (BMI, schizophrenia, educational attainment, externalizing, addiction risk factor, and ADHD) as representative phenotypes from cluster analysis based on global genetic correlations (Supplementary Figure 15).
All traits except ADHD shared common biological processes with DD (Fig. 3). DD shared the greatest number of biological processes with educational attainment (22), followed by BMI (17), externalizing (13), schizophrenia (6), and addiction risk factor (1). No single biological process was shared among all 5 traits. Rather, there was some specificity in the traits represented in different processes. Educational attainment and externalizing overlapped with DD on 11 processes. We found 3 metabolic processes shared between DD and BMI (i.e., “Cobalamin metabolic process”, “Glycosylceramide metabolic process”, “3’-phosphoadenosine 5’-phosphosulfate metabolic process”). These metabolic processes associated with DD were also shared with schizophrenia, externalizing, and educational attainment, highlighting the overlapping mechanisms that contribute to multiple traits simultaneously. Otherwise, there was some degree of specificity in DD biological processes shared only with one other trait, such as schizophrenia, externalizing, educational attainment, or BMI (but none exclusively shared with addiction risk factor; Fig. 3).
Related traits include body mass index (BMI), schizophrenia (SCZ), educational attainment (EA), externalizing (EXT), addiction risk factor (ARF), and attention deficit hyperactivity disorder (ADHD; not shown). The DD systems hierarchy was generated by propagating MAGMA-identified genes in HumanNet-XC, which is a gene interaction network, clustered into gene communities at various resolutions, and annotated using Gene Ontology biological processes. The upper panel shows select systems enriched with at least one of the 6 traits. The node size indicates the number of genes in each system, and the node color indicates the enriched trait(s). The lower panel shows a heatmap of odds ratio for DD and each of the 6 traits, calculated for each process shown in the upper panel. No processes implicated in DD were enriched with ADHD-associated genes. See Supplementary Table 13 for full results.
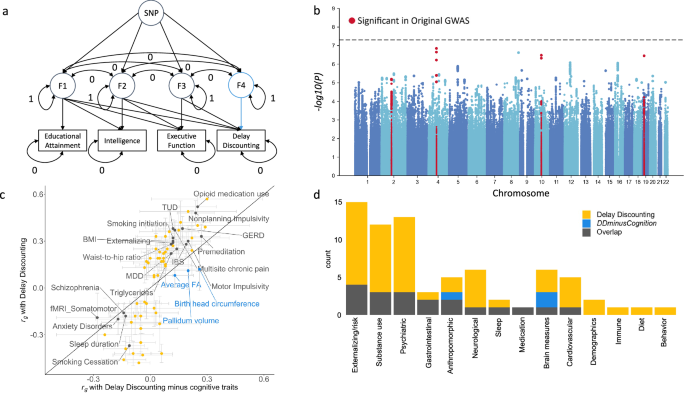
GWAS-by-subtraction identified persistent genetic correlations after accounting for educational attainment, intelligence, and executive function
DD is known to be negatively phenotypically correlated with cognitive traits like educational attainment and IQ [62,63,64]. Accordingly, our global and local genetic correlations both identified negative genetic correlations between cognitive traits and DD. We used the GWAS-by-subtraction [65] approach within genomic structural equation modeling (Genomic SEM) [66] to generate GWAS summary statistics (“DDminusCognition”) that excluded genetic variance related to educational attainment [43] (N = 765,283), intelligence [47] (N = 269,867), and executive function [67] (N = 427,037; Fig. 4A). While the DDminusCognition GWAS did not yield any significant loci, 7 out of the original 11 (63.64%) genome-wide significant loci remained significant at a suggestive threshold (P < 5.00 × 10−05; Fig. 4B; Supplementary Table 14). GWAS signals were most attenuated by accounting for educational attainment, the GWAS with the largest sample size (Supplementary Figure 16). We repeated the global genetic correlation analysis, and found that 19 (27.14%) of the 70 original genetic correlations with DD persisted after accounting for cognitive-related factors (Fig. 4C, D; Supplementary Table 15). These include significant genetic correlations with a broad range of traits and categories, such as smoking (i.e., smoking initiation rg = 0.12 ± 0.03, TUD rg = 0.13 ± 0.04, smoking cessation rg = −0.11 ± 0.04), impulsivity (i.e., premeditation rg = 0.27 ± 0.05, nonplanning impulsivity rg = 0.24 ± 0.05, motor impulsivity rg = 0.20 ± 0.06), externalizing (rg = 0.12 ± 0.04), psychiatric disorders (i.e., SCZ rg = −0.14 ± 0.04, major depressive disorder rg = 0.14 ± 0.04), anthropomorphic measures (i.e., BMI rg = 0.12 ± 0.03, waist-to-hip ratio rg = 0.11 ± 0.03), brain measures (i.e., somatomotor functional connectivity rg = −0.28 ± 0.11), gastrointestinal traits (i.e., gastro-esophageal reflux disease rg = 0.17 ± 0.05, irritable bowel syndrome rg = 0.19 ± 0.06), triglycerides (rg = 0.11 ± 0.04), prescription opioid use (rg = 0.25 ± 0.05), multisite chronic pain (rg = 0.13 ± 0.04), and sleep duration (rg = −0.13 ± 0.04). However, many other substance use (e.g., age of smoking initiation, opioid use disorder), psychiatric (e.g., ADHD, bipolar disorder, anorexia nervosa, obsessive-compulsive disorder), externalizing-related traits (e.g., age at first birth, age at first sex), neurological (e.g., migraine, hip pain), and cardiovascular (e.g., coronary artery disease) traits, among others, were not genetically correlated with DD following adjustment for cognitive-related factors (Fig. 4C, D). Educational attainment had the largest impact on the change in genetic correlation between the original and GWAS-by-subtraction DD GWASs (Supplementary Table 15).
a Cholesky model structure showing path estimates for a single SNP fitted using genomic SEM. Educational attainment, intelligence, executive function, DD, and SNP are observed variables; F1-4 are latent (unobserved) variables. F4 represents DDminusCognition. The covariances between all the latent variables are constrained to 0. The residual variances of educational attainment, intelligence, executive function, and DD are constrained to 0, so that all variance is explained by the latent factors. The variances of the latent factors are constrained to 1. b Manhattan plot for the DDminusCognition GWAS. The upper dashed line denotes the genome-wide significance threshold (P = 5.00 × 10−08) and lower dashed line indicates nominal significance threshold (P = 5.00 × 10−05). Red dots indicate SNPs that were significant in the original DD GWAS. c DD genetic correlations (rg ± standard error) exclusively significant in the original GWAS (yellow), the DDminusCognition GWAS (blue), or significant in both GWAS (gray; Supplementary Table 16). d Number of significant genetic correlations per trait category that were exclusive to the original GWAS, the DDminusCognition GWAS, or significant in both.
DD polygenic score was associated with 212 medical outcomes
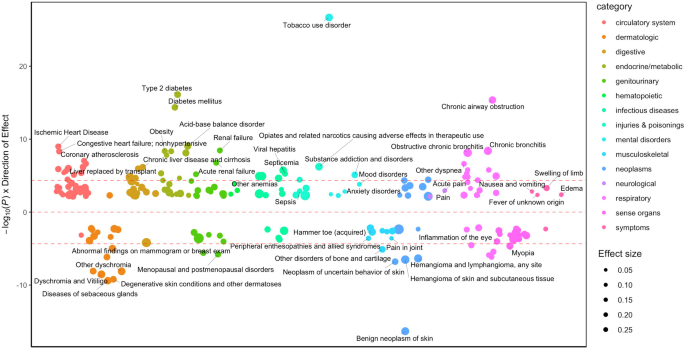
To extend the associations with DD to medical outcomes, we performed a PheWAS of DD polygenic scores (PGS) against 1,318 diagnostic outcomes in the BioVU medical cohort (NEuropean = 66,917; NAfrican = 12,383). In the European cohort, DD PGS was associated with 212 medical outcomes. We identified expected associations with 2 substance-related diagnoses (i.e., TUD OR = 1.14, CI 95%: 1.11-1.16, substance addiction and disorders OR = 1.09, CI 95%: 1.05-1.13) and 4 psychiatric disorders (e.g., mood disorders OR = 1.04, CI 95%: 1.02-1.06). We also found associations with 24 respiratory (e.g., chronic airway obstruction OR = 1.08, CI 95%: 1.04-1.11), 17 sense organs (e.g., myopia OR = 0.90, CI 95%: 0.85-0.95), and 36 circulatory (e.g., ischemic heart disease OR = 1.04, CI 95%: 1.02-1.07) conditions. Consistent with our genetic correlation and network analyses, we also found that DD PGS associated with 18 endocrine/metabolic conditions (e.g., Type 2 diabetes OR = 1.10, CI 95%: 1.07-1.13, obesity OR = 1.09, CI 95%: 1.04-1.13; Fig. 5, Supplementary Table 16). Considering that we identified robust associations with smoking, we performed a sensitivity analysis controlling for TUD diagnosis. Some associations persisted (e.g., sense organs, dermatologic traits, neoplasms) whereas other associations were attenuated (e.g., circulatory, respiratory, endocrine/metabolic) or nullified (e.g., psychiatric disorders; Supplementary Figure 17, Supplementary Table 16).
Only FDR-significant results with 212 medical outcomes are annotated (see Supplementary Table 16 for full results). Red dotted lines show the Bonferroni significance threshold. The size of the circles denotes effect size, with larger circles indicating stronger effects.
There was some specificity in DD PGS associations across age groups (Supplementary Table 16). In the 19-25 age group (N = 6,241-7,246), we identified 2 associations, including positive associations with pregnancy complications (i.e., early or threatened labor; hemorrhage in early pregnancy OR = 1.30, CI 95%: 1.19-1.41, other conditions or status of the mother complicating pregnancy, childbirth, or the puerperium OR = 1.30, CI 95%: 1.18-1.42). In the 41–60 age group (N = 26,269-31,381), we identified 133 associations, including positive associations with substance use and psychiatric disorders (e.g., TUD OR = 1.17, CI 95%: 1.14-1.21, major depressive disorder OR = 1.09, CI 95%: 1.04-1.14) and physical health conditions (e.g., diabetes mellitus OR = 1.12, CI 95%: 1.09-1.15, obesity OR = 1.11, CI 95%: 1.07-1.15, renal failure OR = 1.11, CI 95%: 1.07-1.14). In the 61–100 age group (N = 22,405-26,855), we identified 44 associations, primarily positive associations with cardiovascular conditions (25.00% of all associations; e.g., myocardial infarction OR = 1.11, CI 95%: 1.06-1.15, ischemic heart disease OR = 1.06, CI 95%: 1.03-1.09). The only psychiatric association in this age group was with TUD (OR = 1.12, CI 95%: 1.08-1.17). We did not observe age-specific associations in the 0–11 (N = 7,042-8,459), 12-18 (N = 5,886-6,918), and 26–40 groups (N = 13,261-15,455), perhaps due to lower power due to smaller sample sizes.
We did not find any significant PheWAS associations in the African cohort (Supplementary Table 17), presumably due to both lower statistical power (N = 7,081-12,308) and the poor performance of our PGS, which were based on a European cohort. Portability of PGS decreases with greater genetic distance from the discovery sample, which is a well-known limitation [68].
Discussion
We expanded our prior GWAS by over 5-fold [21], leading to the identification of 11 independent loci and 93 candidate risk genes associated with DD. DD was genetically correlated with numerous psychiatric, neurobiological, and physical health outcomes; we identified 3 pleiotropic hotspots and biological processes underlying these associations. Although DD is an aspect of cognition, multivariate analyses identified genetic variation in DD that is not explained by broader cognitive-related measures. In a hospital cohort, DD PGS was associated with a multitude of medical outcomes, many of which may be related to health consequences of disadvantageous behaviors related to DD. This work pinpoints neurobiological targets of DD and sets the foundation for future studies that may enable the discovery of better prevention, diagnosis, and treatment mechanisms for a host of conditions.
The increased power from our current GWAS [21] allowed us to identify 11 novel loci and 93 genes associated with DD. We identified 4 genes that were implicated by all 3 gene-based and transcriptome-based methods: SULT1A1, SH2B1, TUFM, and NPIPB6. These genes are highly pleiotropic; they have been implicated in risk-taking [27], substance use [29, 69, 70], intelligence and cognitive ability [46, 71], obesity and BMI [53, 69, 72,73,74,75,76,77,78,79,80,81,82,83], and brain morphometrics [84,85,86]. SULT1A1 encodes the SULT1A1 sulfotransferase enzyme that is induced by dopamine in neuroepithelial cells in vitro [87, 88]. The role of dopaminergic neurotransmission on DD is well-studied [89, 90]. SH2B1 encodes the SH2B1 adaptor protein, which mediates the activation of various kinases and is implicated in neurite outgrowth [91,92,93,94], neuron differentiation and brain growth [92, 95], and obesity-related phenotypes [96,97,98,99]. Recent GWAS [95] and candidate gene [95, 100] studies implicated SH2B1 variants in fluid intelligence, aggression, and brain growth, which are partially corroborated by deletion of Sh2b1 in mice [95, 100]. TUFM encodes the Tu Translation Elongation Factor, Mitochondrial protein. It is hypothesized that proteins maintaining mitochondrial synthesis like TUFM mediate synapse development, function, and plasticity that ultimately impact cognition and executive functions [101]. The function of NPIPB6 is not defined, though one transcriptome-wide association study found that its downregulation in the basal ganglia is associated with intelligence-associated variants [102]. These four genes are concentrated in the chr16p11.2 locus, which has been implicated in many of the traits associated with DD, including psychiatric disorders (e.g., autism spectrum disorder, ADHD, schizophrenia, bipolar disorder) [103], BMI and eating behaviors [104,105,106,107,108], head size and brain volume [106,107,108,109], and intellectual and cognitive ability [105, 107, 110], most notably inhibitory control [106]. Notably, we did not replicate our previously reported association with rs6528024 [21]. There are numerous other examples of genome-wide significant loci not being replicated (e.g., [111]). Given the approximately 5-fold larger sample in the current study, this suggests that the original finding may have been a false positive. It is important to note that rs6528024 has a low MAF and is on the X-chromosome. Furthermore, while we log10-transformed mean k values, this transformation does not produce a perfectly normal distribution. All of these factors could have made a false positive more likely. However, a mouse model in which the nearest gene to rs6528024 (GPM6B) was deleted showed behavioral differences related to impulsivity [112], which could be taken to corroborate the original finding.
We found that DD is genetically correlated with a constellation of other traits from multiple categories. This included genetic correlations with brain-related traits that are consistent with neuroimaging studies showing associations between limbic and somatomotor connectivity and DD in adults [113,114,115,116] and children [117]. These genetic correlations may arise from unique combinations of gene sets and processes that are trait-specific, as reflected in our network analysis. For example, although ADHD, externalizing behaviors, and addiction share behavioral and genetic risk factors, the specific genetic architecture of DD may be distinct. While externalizing and addiction traits often involve impulsive decision-making, DD specifically captures temporal discounting, which may engage different neurobiological pathways [118]. The divergence in genetic architecture suggests that while these traits co-occur, they may be driven by overlapping but non-identical biological mechanisms. This observation emphasizes the potential benefit of exploring multiple facets of impulsivity, including DD.
We found similar broad-ranging associations between DD PGS and psychiatric (e.g., addiction, mood) and physical (e.g., cardiovascular, endocrine/metabolic, pain) outcomes, many of which could be the result of behaviors related to DD, such as smoking. These findings mirror associations found across decades of epidemiological studies [8, 14, 64, 119, 120]. One of our most consistent observations was that DD was genetically correlated with various smoking traits, from aspects of smoking initiation to cessation. Prior studies indicate that steeper DD associates with relapse, especially for smoking [121], which could suggest that pharmacologically or behaviorally targeting DD could enhance SUDs treatment response [122,123,124], although we were unable to identify pharmacotherapies that target genes implicated in DD via drug repositioning (Supplementary Tables 18, 19). We also noted that liability for steeper DD was not always disadvantageous. For example, we identified negative genetic correlations with obsessive-compulsive disorder, anorexia nervosa, autism spectrum disorder, schizophrenia, and bipolar disorder. Likewise, we identified negative associations between DD PGS and associations with some dermatological, musculoskeletal, and sense organ conditions. Some of these unexpected correlations, such as the negative genetic correlations between DD and schizophrenia, align with our previous findings [21] and suggest that shared genetic factors influence these traits in opposing directions. Some of these genetic correlations contradict prior phenotypic findings showing positive phenotypic correlations between DD and schizophrenia [10, 13, 14] and bipolar disorder [10]. While phenotypic correlations are estimated within the same individuals and reflect both genetic and environmental influences, genetic correlations are typically estimated across individuals from independent cohorts and capture genetic but not environmental factors. This discrepancy highlights the need to disentangle genetic and environmental influences to better understand the relationship between DD and other complex traits.
To date, the etiological factors underlying the association of DD with psychiatric and physical health outcomes are not known. Here, we report local genetic correlations that provide candidate regions contributing to pleiotropy. We identified a hotspot on chr6q16.1, in which we observed positive and negative genetic correlations between DD and 8 psychiatric, cognitive, and physical traits. In other instances, we observed traits that were globally genetically correlated with DD but showed only one local genetic correlation. For example, the positive global genetic correlation with addiction risk factor was recapitulated at the chr11q23.1-2 locus that includes the NCAM1-TTC12-ANKK1-DRD2 gene cluster, which is well-known for its association with reward processes and psychiatric conditions, including substance use disorders [125,126,127,128,129,130,131,132,133,134,135,136]. DD hotspots often span broad regions encompassing dozens of genes; DD can be measured in non-human animals [112, 137, 138], which can be used to validate and parse some of these complex findings.
DD is part of a family of cognitive [62,63,64] and executive function processes, including impulsivity [5]. While we observed genetic correlations between DD and educational attainment, intelligence, and executive function, as well as several impulsivity facets, these constructs are dissociable at a genetic level. For example, cluster analysis identified that DD loaded onto a factor discrete from other impulsivity measures [139,140,141,142], and we found through genetic correlation, network, and GWAS-by-subtraction analyses that DD only partially overlaps with educational attainment, intelligence, and executive function. Educational attainment was most strongly genetically correlated with DD. Although this may be explained in part by increased statistical power with the larger sample size, it is also consistent with the idea that educational attainment greatly requires shallow DD (e.g., working toward delayed rewards and long-term goal-setting [62]) or other non-cognitive factors that are negatively genetically correlated with DD [65].
DD PGS PheWAS corroborated numerous positive associations with health outcomes observed by prior phenotypic studies [6, 9, 10, 12, 13, 16,17,18] that were also consistent with our genetic correlations. For example, we identified significant associations with several chronic pain conditions (Fig. 5, Supplementary Figure 15), which may reflect common underlying mechanisms related to dopaminergic and opioid systems in both traits. Age-stratified PheWAS revealed associations that were missed in the original analysis using the full cohort (e.g., pregnancy complications in the 19–25 group), but overall effect sizes for PheWAS associations were largely consistent across age groups, in line with prior observations that DD expression and heritability stabilizes by adolescence [2, 19, 20, 143]. The persistence of some DD PGS associations with these outcomes, even after accounting for the co-occurrence of other adverse health conditions related to DD (e.g., adjusting for TUD), asserts that many of these relationships are influenced by both biological and behavioral factors.
Our findings have several limitations. First, there may be a cohort bias in discounting rates since 23andMe research participants are a self-selected group that must first join 23andMe and then agree to participate in an uncompensated research study. These research participants tend to be more educated, older, and have a higher socioeconomic status compared to the general population [144]. We included age as a covariate in our analyses but not educational attainment [43], since including heritable traits as covariates may introduce collider bias [145]. Education was accounted for in the GWAS-by-subtraction analysis, albeit at a substantial loss in power to detect significant loci unique to DD. Second, these analyses do not address causality between DD and other traits, which may be complex and bidirectional in some cases. For example, substance use has been shown to increase DD, yet steeper discounting rates also predict substance use [6]. We suspect SUDs play a minimal role on DD in our population because the 23andMe research cohort that we studied did not heavily engage in substance use [141, 146]. Our investigations are correlational in nature and may be influenced by many other unmeasured factors, such as cross-trait assortative mating [147]; future studies using causality inference techniques and longitudinal analyses could clarify the direction of these associations. Similarly, DD can be greatly affected by environmental factors, including but not limited to socioeconomic status. The strong genetic correlations between DD and environmental influences, such as educational attainment, underscore the importance of considering environmental moderators in understanding the complex etiology of DD in future studies [148]. Finally, our results indicate that DD is highly polygenic. However, each genome-wide significant variant confers only a small effect of DD. Novel associations, especially those with no prior support from other psychiatric GWAS, need further replication. GWAS were only conducted in individuals most genetically similar to European reference populations due to the large sample size required to detect the small effect of individual variants, and future analyses should diversify genetic analyses as larger non-European samples become available.
DD is a fundamental cognitive process involved in daily life that is broadly related to psychopathology and can be easily evaluated in health and clinical populations [2]. Our findings show that DD shares overlapping biological underpinnings with numerous psychiatric, cognitive, and physical outcomes, which may help propel prevention and treatment strategies across a broad spectrum of human health.
Methods
Sample and DD assessment
All individuals included in this study were research participants from 23andMe, as previously described [21, 142]. Participants provided informed consent and participated in the research online, under a protocol approved by the external Association for the Accreditation of Human Research Protection Programs (AAHRPP)-accredited Institutional Review Board (IRB), Ethical & Independent (E&I) Review Services (http://www.eandireview.com/). As of 2022, E&I Review Services is part of Salus IRB (https://www.versiticlinicaltrials.org/salusirb).
Participants completed the online 27-item MCQ as part of a larger survey to assess preference for smaller, immediate rewards versus larger, delayed rewards (see Supplementary Methods for questionnaire). The overall response pattern was used to derive temporal discounting functions (k), wherein higher values indicate a greater devaluation of delayed rewards (i.e., preference for immediate gratification). Participants with low response concordance or inappropriate response rates were excluded from the analysis (Supplementary Methods). k was not normally distributed; all further analysis was conducted on log10(k) values [26].
Genome-wide association study
We borrowed population descriptors recommended in a recent report by the National Academies of Sciences, Engineering, and Medicine to define our study cohorts [22]. GWAS included 134,935 unrelated 23andMe US-based participants with at least 97% European ancestry, as determined through an analysis of local ancestry (Supplementary Methods). DNA extraction and genotyping were performed on saliva samples at clinical laboratories of Laboratory Corporation of America, certified by the Clinical Laboratory Improvement Amendments (CLIA) and accredited by the College of American Pathologists (CAP). Quality control, imputation, and genome-wide analysis were conducted by 23andMe (Supplementary Methods), as previously described [21]. A total of 14,137,232 SNPs passed the GWAS quality control (Supplementary Table 3). Association tests were conducted via linear regression under an additive model using a proprietary pipeline developed internally by 23andMe (P < 5.00 × 10−08). We included age (inverse-normal transformed), sex, the top five principal components of genotype, and indicator variables for genotype platforms as covariates. We identified previous associations with the SNPs linked to DD based on GWAS catalog information [149].
Gene- and transcriptome-based analyses
We conducted bioannotation and bioinformatic analyses to further characterize the loci identified by the DD GWAS. Gene-set analysis was conducted using Multi-marker Analysis of GenoMic Annotation (MAGMA; v1.08) [54] through the FUMA (v1.3.6a) web-based platform [150], with significance determined at a Bonferroni threshold based on the total number of genes tested (P < 2.53 × 10−06). We used Hi-C coupled MAGMA (H-MAGMA) [55, 56] to assign intergenic and intronic SNPs to genes based on their chromatin interactions based on 4 Hi-C datasets. We applied a Bonferroni correction based on the total number of gene-tissue pairs tested (P < 9.78 × 10−07). We used S-PrediXcan to identify expression quantitative trait loci-linked genes across 13 brain tissues associated with DD. We applied a Bonferroni threshold based on the number of genes per tissue (N = 2,532–6,744; P < 1.97 × 10−05 to 7.41 × 10−06). See Supplementary Methods for more details on gene- and transcriptome-based analysis.
Heritability and genetic correlation analyses
We used LDSC [23, 24] to calculate SNP-based heritability from common SNPs mapped to HapMap3 data [151] using pre-computed linkage disequilibrium scores (“eur_w_ld_chr/”). We also used LDSC to estimate genetic correlations between DD and 109 other traits. Traits were selected based on previously known phenotypic associations between DD and related traits (e.g., SUDs, impulsivity measures, metabolic traits). LDSC estimates the genetic correlation between complex traits or diseases by leveraging summary statistics from GWAS and patterns of linkage disequilibrium across the genome. We applied a 5% FDR threshold to account for multiple testing.
Local genetic correlation
While global genetic correlations measure the overall genetic similarity between two traits across the entire genome, local genetic correlations assess genetic similarity between traits within specific genomic regions. Notably, local genetic correlations can identify specific overlapping loci, even if the global correlation is weak or absent. To estimate the “local” rg between DD and the 109 traits used in LDSC, we used LAVA [25]. LAVA splits the genome into 2,495 non-overlapping blocks of approximately equal size (~1 Mb) while minimizing the LD between them and estimates the bivariate SNP genetic correlation between 2 phenotypes for each block. LAVA first runs univariate tests to determine the amount of local genetic signal for all traits of interest and filters out loci with non-significant heritability for each trait. It then performs bivariate tests to obtain the local genetic correlation between pairs of traits with significant heritability (P < 2.00 × 10−05). We further corrected the bivariate analysis using FDR correction.
Network propagation and visualization
We modified the Python package NetColoc [152,153,154] for network propagation of a single gene set. The genes associated with DD in MAGMA analysis were used as “seed” genes. For network propagation, we used a Random Walk with Restart algorithm [155], simulating heat spreading from the “hot” seed genes to adjacent genes in the network. The total heat within the system is conserved by dissipating a constant fraction of heat from each gene with each iteration. The process of heat diffusion stabilizes at a steady-state solution, as detailed in Equation 1:
$$F={\left(I-\alpha W\right)}^{-1}(1-\alpha ){\gamma }_{0}$$
Where F represents the final heat distribution across nodes, Y0 is the vector of seed genes, W is the normalized adjacency matrix (HumanNet), and α (between 0 and 1) is the heat dissipation rate. After network propagation with α = 0.5, a network proximity score (NPS) was calculated for each gene by comparing the observed results to the expected results. The expected results were generated by propagating 100 random seed gene-sets, each of which was sampled to preserve the size and degree distribution of the original seed gene-set [156]. For each gene g, NPS was calculated as a Z score, comparing its observed heat Fg,S after propagation to the average expected heat Fg, and from the random sets, as outlined in Equation 2:
$${{NPS}}_{g,S}=\frac{log log ({F}_{g,S})-{{\langle }}log log ({\vec{F}}_{g,{rand}}){{\rangle }}}{\sigma (log log ({\vec{F}}_{g,{rand}}))}$$
Where ⟨ ⟩ denotes the mean of a vector, and σ denotes the standard deviation of a vector. All heat values are log-transformed to ensure they follow a normal distribution. To select for genes highly proximal to seed genes, we filtered for those with NPS > 3.
We built a multiscale DD network map using the Hierarchical community Decoding Framework (HiDeF) algorithm from Cytoscape’s CDAPS package [157]. HiDef employs persistent homology for simultaneous multi-scale community detection. The interaction network is translated into a fully connected similarity network, and then network communities are detected at various modularity levels, from smaller to larger communities by varying resolutions. Communities consistently appearing across resolutions were determined using Jaccard similarities and subsequently organized hierarchically, with their relationships assessed by the containment index (CI), which quantifies the overlap between communities v and w: If CI (v, w) exceeds the threshold σ, an edge is added from v to w, and any redundant edges are reduced in the final hierarchy. For the DD systems map, we used HiDeF with the maximum resolution at 5, with all other parameters set at their default values. The resulting communities were first annotated based on Gene Ontology (GO) Biological Processes using Enrichr [158] and then manually refined. Gene enrichment for educational attainment, addiction risk factor, schizophrenia, externalizing, BMI, and ADHD was evaluated for each community using a hypergeometric test, followed by FDR correction. All hierarchical and network diagrams were created with Cytoscape [159].
GWAS-by-subtraction
We used the genomic SEM (v0.0.5) R package [66] and published GWAS of educational attainment [43] (N = 765,283), intelligence [47] (N = 269,867), and executive function [67] (N = 427,037) to estimate SNP associations with DD that are independent of associations with cognitive-related traits. The model regressed GWAS summary statistics of educational attainment, intelligence, executive function, and DD onto 4 latent factors (F1-4), which were restrained to be uncorrelated (Fig. 4a). Latent factors were regressed on each SNP and a GWAS of F4, which represents the genetic effects on DD that remained after accounting for the other traits (“DDminusCognition”), was conducted. We computed the genetic correlations within genomic SEM wherein a similar model as mentioned above was fitted, except the latent factors were regressed on the target trait instead of each SNP. This yielded a genetic correlation between the target trait and the variance in DD that is not overlapping with other cognitive-related traits.
Phenome-wide association study
We conducted a PheWAS with DD PGS using electronic health record (EHR) data from unrelated participants (NEuropean = 66,917; NAfrican = 12,383) of the Vanderbilt University Medical Center (VUMC)’s biobank, BioVU (IRB #160302, #172020, #190418) [160]. We computed PGS for DD using PRS-Continuous shrinkage software (PRS-CS) [161] using default settings to estimate shrinkage parameters and random seed fixed for reproducibility. For 1,817 medical conditions available through BioVU, we defined cases as patients who received at least two International Disease Classification (ICD, ICD-9 or ICD-10) diagnostic codes (also known as “phecodes”) in their EHR, and controls as patients with no diagnostic codes for that condition. To map ICD codes to phecodes, we used Phecode Map 1.2. Logistic models were fitted for each of the phecodes using the PheWAS v0.12 R package [162] adjusting for sex, median age of longitudinal EHR, and the first ten principal components. A minimum of 100 cases were required for phecode inclusion, leaving a total of 1,318 phecodes. Sensitivity analyses were conducted using TUD status (phecode 318) as a covariate. To examine potential age-specific associations with health outcomes, independent PheWAS analyses were conducted across 6 distinct age bins: 0–11, 12–18, 19–25, 26–40, 41–60, and 61–100. We used a 5% FDR threshold to correct for multiple testing.
Drug repositioning
We used DRUGSETS [163] to facilitate genetically informed drug repositioning through drug gene-set analysis using MAGMA [54]. The data for drug-gene targets and interactions were drawn from the Clue Repurposing Hub and the Drug Gene Interaction Database. Gene-sets were created for 1,201 drugs comprising genes whose protein products are targeted by or interact with that specific drug. Competitive gene-set analysis was performed using the MAGMA software tool while conditioning on a gene-set of all drug target genes in the data (N = 2,281) to assess whether significant associations were due to effects unique to drug pathways or common properties of drug target genes. A Bonferroni significance threshold was applied based on the number of drug-gene sets analyzed (N = 735; P < 6.80 × 10−05). Additionally, drug gene-sets were categorized based on Anatomical Therapeutic Classification (ATC) III code, clinical indication, and mechanism of action. Each drug gene-set group with 5 or more drugs within the ATC (N = 63), mechanism of action (N = 51), and clinical indication (N = 80) categories was tested for enrichment of DD associated genes using a Bonferroni significance threshold (ATC III P < 7.94 × 10−04, clinical indication P < 6.25 × 10−04, mechanism of action P < 9.80 × 10−04).
Data availability
We provide 23andMe summary statistics for the top 10,000 SNPs (see Supplementary Information). The full 23andMe GWAS summary statistics will be made available through 23andMe to qualified researchers under an agreement with 23andMe that protects the privacy of the 23andMe participants. Please visit https://research.23andme.com/collaborate/#dataset-access/ for more information and to apply to access the data. The following resources were used for secondary analysis: FUMA (https://fuma.ctglab.nl/), Ensembl build 85 (https://www.ebi.ac.uk/about/news/updates-from-data-resources/ensembl-version-85/), 1000 Genomes Project phase 3 (https://internationalgenome.org/data-portal/sample), Msigdb v.7.0 (https://data.broadinstitute.org/gsea-msigdb/msigdb/release/7.0/), Genotype–Tissue Expression (GTEx) v.8 project database (https://www.gtexportal.org/), H-MAGMA software and Hi-C datasets (https://github.com/thewonlab/H-MAGMA), PredictDB Data Repository (http://predictdb.hakyimlab.org/ and http://predictdb.org/), BrainQTL (http://predictdb.hakyimlab.org/), local LDSC (https://github.com/bulik/ldsc), NetColoc (https://pypi.org/project/netcoloc/), and Phecode Map 1.2 (https://phewascatalog.org/phecodes).
References
Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87.
Lempert KM, Steinglass JE, Pinto A, Kable JW, Simpson HB. Can delay discounting deliver on the promise of RDoC? Psychol Med. 2019;49:190–9.
Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15:227–39.
Caswell AJ, Bond R, Duka T, Morgan MJ. Further evidence of the heterogeneous nature of impulsivity. Pers Individ Dif. 2015;76:68–74.
Madden GJ, Bickel WK. Impulsivity: The behavioral and neurological science of discounting. American Psychological Association; 2010.
Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134:287–97.
MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl). 2011;216:305–21.
Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction. 2017;112:51–62.
Weinsztok S, Brassard S, Balodis I, Martin LE, Amlung M. Delay discounting in established and proposed behavioral addictions: a systematic review and meta-analysis. Front Behav Neurosci. 2021;15:786358.
Amlung M, Marsden E, Holshausen K, Morris V, Patel H, Vedelago L, et al. Delay discounting as a transdiagnostic process in psychiatric disorders: a meta-analysis. JAMA Psychiatry. 2019;76:1176–86.
Bickel WK, Freitas-Lemos R, Tomlinson DC, Craft WH, Keith DR, Athamneh LN, et al. Temporal discounting as a candidate behavioral marker of obesity. Neurosci Biobehav Rev. 2021;129:307–29.
Grimm O, van Rooij D, Hoogman M, Klein M, Buitelaar J, Franke B, et al. Transdiagnostic neuroimaging of reward system phenotypes in ADHD and comorbid disorders. Neurosci Biobehav Rev. 2021;128:165–81.
Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12:213–21.
Amlung M, Petker T, Jackson J, Balodis I, MacKillop J. Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychol Med. 2016;46:2423–34.
Bird BM, Levitt EE, Stewart SH, Wanklyn SG, Meyer EC, Murphy JG, et al. Posttraumatic stress and delay discounting: a meta-analytic review. Psychol Med. 2024;54:437–46.
Pinto A, Steinglass JE, Greene AL, Weber EU, Simpson HB. Capacity to delay reward differentiates obsessive-compulsive disorder and obsessive-compulsive personality disorder. Biol Psychiatry. 2014;75:653–9.
Schuman I, Wang J, Ballard IC, Lapate RC. Waiting for it: anorexia risk, future orientation, and intertemporal discounting. Res Sq. 2024:rs.3.rs-4002723.
Stern CM, McPherson I, Dreier MJ, Coniglio K, Palmer LP, Gydus J, et al. Avoidant/restrictive food intake disorder differs from anorexia nervosa in delay discounting. J Eat Disord. 2024;12:19.
Anokhin AP, Golosheykin S, Grant JD, Heath AC. Heritability of delay discounting in adolescence: a longitudinal twin study. Behav Genet. 2011;41:175–83.
Anokhin AP, Grant JD, Mulligan RC, Heath AC. The genetics of impulsivity: evidence for the heritability of delay discounting. Biol Psychiatry. 2015;77:887–94.
Sanchez-Roige S, Fontanillas P, Elson SL, Pandit A, Schmidt EM, Foerster JR, et al. Genome-wide association study of delay discounting in 23,217 adult research participants of European ancestry. Nat Neurosci. 2018;21:16–8.
Using Population Descriptors in Genetics and Genomics Research: A New Framework for an Evolving Field. Washington (DC); 2023.
Bulik-Sullivan B, Finucane HK, Anttila V, Gusev A, Day FR, Loh P-R, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet. 2015;47:1236–41.
Bulik-Sullivan BK, Loh P-R, Finucane HK, Ripke S, Yang J, Patterson N, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291–5.
Werme J, van der Sluis S, Posthuma D, de Leeuw CA. An integrated framework for local genetic correlation analysis. Nat Genet. 2022;54:274–82.
Gray JC, Amlung MT, Palmer AA, MacKillop J. Syntax for calculation of discounting indices from the monetary choice questionnaire and probability discounting questionnaire. J Exp Anal Behav. 2016;106:156–63.
Karlsson Linnér R, Biroli P, Kong E, Meddens SFW, Wedow R, Fontana MA, et al. Genome-wide association analyses of risk tolerance and risky behaviors in over 1 million individuals identify hundreds of loci and shared genetic influences. Nat Genet. 2019;51:245–57.
Baselmans B, Hammerschlag AR, Noordijk S, Ip H, van der Zee M, de Geus E, et al. The genetic and neural substrates of externalizing behavior. Biol Psychiatry Glob Open Sci. 2022;2:389–99.
Saunders GRB, Wang X, Chen F, Jang S-K, Liu M, Wang C, et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature. 2022;612:720–4.
Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet. 2019;51:237–44.
Pasman JA, Demange PA, Guloksuz S, Willemsen AHM, Abdellaoui A, Ten Have M, et al. Genetic risk for smoking: disentangling interplay between genes and socioeconomic status. Behav Genet. 2022;52:92–107.
Brazel DM, Jiang Y, Hughey JM, Turcot V, Zhan X, Gong J, et al. Exome chip meta-analysis fine maps causal variants and elucidates the genetic architecture of rare coding variants in smoking and alcohol use. Biol Psychiatry. 2019;85:946–55.
Said MA, van de Vegte YJ, Verweij N, van der Harst P. Associations of observational and genetically determined caffeine intake with coronary artery disease and diabetes mellitus. J Am Heart Assoc. 2020;9:e016808.
Thorpe HHA, Fontanillas P, Pham BK, Meredith JJ, Jennings MV, Courchesne-Krak NS, et al. Genome-wide association studies of coffee intake in UK/US participants of European ancestry uncover cohort-specific genetic associations. Neuropsychopharmacology. 2024;49:1609–18.
Hou L, Bergen SE, Akula N, Song J, Hultman CM, Landén M, et al. Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum Mol Genet. 2016;25:3383–94.
Li H-J, Zhang C, Hui L, Zhou D-S, Li Y, Zhang C-Y, et al. Novel risk loci associated with genetic risk for bipolar disorder among han chinese individuals: a genome-wide association study and meta-analysis. JAMA Psychiatry. 2021;78:320–30.
Mühleisen TW, Leber M, Schulze TG, Strohmaier J, Degenhardt F, Treutlein J, et al. Genome-wide association study reveals two new risk loci for bipolar disorder. Nat Commun. 2014;5:3339.
Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–29.
Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803.
Karlsson Linnér R, Mallard TT, Barr PB, Sanchez-Roige S, Madole JW, Driver MN, et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat Neurosci. 2021;24:1367–76.
Luciano M, Hagenaars SP, Davies G, Hill WD, Clarke T-K, Shirali M, et al. Association analysis in over 329,000 individuals identifies 116 independent variants influencing neuroticism. Nat Genet. 2018;50:6–11.
Wendt FR, Pathak GA, Singh K, Stein MB, Koenen KC, Krystal JH, et al. Sex-specific genetic and transcriptomic liability to neuroticism. Biol Psychiatry. 2023;93:243–52.
Okbay A, Wu Y, Wang N, Jayashankar H, Bennett M, Nehzati SM, et al. Polygenic prediction of educational attainment within and between families from genome-wide association analyses in 3 million individuals. Nat Genet. 2022;54:437–49.
Rietveld CA, Medland SE, Derringer J, Yang J, Esko T, Martin NW, et al. GWAS of 126,559 individuals identifies genetic variants associated with educational attainment. Science. 2013;340:1467–71.
Davies G, Armstrong N, Bis JC, Bressler J, Chouraki V, Giddaluru S, et al. Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N = 53949). Mol Psychiatry. 2015;20:183–92.
Davies G, Lam M, Harris SE, Trampush JW, Luciano M, Hill WD, et al. Study of 300,486 individuals identifies 148 independent genetic loci influencing general cognitive function. Nat Commun. 2018;9:2098.
Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, de Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50:912–9.
Sniekers S, Stringer S, Watanabe K, Jansen PR, Coleman JRI, Krapohl E, et al. Genome-wide association meta-analysis of 78,308 individuals identifies new loci and genes influencing human intelligence. Nat Genet. 2017;49:1107–12.
Williams CM, Labouret G, Wolfram T, Peyre H, Ramus F. A general cognitive ability factor for the UK biobank. Behav Genet. 2023;53:85–100.
Hill WD, Davies NM, Ritchie SJ, Skene NG, Bryois J, Bell S, et al. Genome-wide analysis identifies molecular systems and 149 genetic loci associated with income. Nat Commun. 2019;10:5741.
Hawkes G, Beaumont RN, Tyrrell J, Power GM, Wood A, Laakso M, et al. Genetic evidence that high BMI in childhood has a protective effect on intermediate diabetes traits, including measures of insulin sensitivity and secretion, after accounting for BMI in adulthood. Diabetologia. 2023;66:1472–80.
Hoffmann TJ, Choquet H, Yin J, Banda Y, Kvale MN, Glymour M, et al. A large multiethnic genome-wide association study of adult body mass index identifies novel loci. Genetics. 2018;210:499–515.
Huang J, Huffman JE, Huang Y, Do Valle Í, Assimes TL, Raghavan S, et al. Genomics and phenomics of body mass index reveals a complex disease network. Nat Commun. 2022;13:7973.
de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol. 2015;11:e1004219.
Sey NYA, Hu B, Mah W, Fauni H, McAfee JC, Rajarajan P, et al. A computational tool (H-MAGMA) for improved prediction of brain-disorder risk genes by incorporating brain chromatin interaction profiles. Nat Neurosci. 2020;23:583–93.
Sey NYA, Pratt BM, Won H. Annotating genetic variants to target genes using H-MAGMA. Nat Protoc. 2023;18:22–35.
Barbeira AN, Bonazzola R, Gamazon ER, Liang Y, Park Y, Kim-Hellmuth S, et al. Exploiting the GTEx resources to decipher the mechanisms at GWAS loci. Genome Biol. 2021;22:49.
Barbeira AN, Dickinson SP, Bonazzola R, Zheng J, Wheeler HE, Torres JM, et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat Commun. 2018;9:1825.
Gamazon ER, Wheeler HE, Shah KP, Mozaffari SV, Aquino-Michaels K, Carroll RJ, et al. A gene-based association method for mapping traits using reference transcriptome data. Nat Genet. 2015;47:1091–8.
Kim CY, Baek S, Cha J, Yang S, Kim E, Marcotte EM, et al. HumanNet v3: an improved database of human gene networks for disease research. Nucleic Acids Res. 2022;50:D632–9.
Wright SN, Colton S, Schaffer LV, Pillich RT, Churas C, Pratt D, et al. State of the Interactomes: an evaluation of molecular networks for generating biological insights. Mol Syst Biol. 2025;21:1–29.
Jaroni JL, Wright SM, Lerman C, Epstein LH. Relationship between education and delay discounting in smokers. Addict Behav. 2004;29:1171–5.
Szuhany KL, MacKenzie DJ, Otto MW. The impact of depressed mood, working memory capacity, and priming on delay discounting. J Behav Ther Exp Psychiatry. 2018;60:37–41.
Wit H, de, Flory JD, Acheson A, McCloskey M, Manuck SB. IQ and nonplanning impulsivity are independently associated with delay discounting in middle-aged adults. Pers Individ Dif. 2007;42:111–21.
Demange PA, Malanchini M, Mallard TT, Biroli P, Cox SR, Grotzinger AD, et al. Investigating the genetic architecture of noncognitive skills using GWAS-by-subtraction. Nat Genet. 2021;53:35–44.
Grotzinger AD, Rhemtulla M, de Vlaming R, Ritchie SJ, Mallard TT, Hill WD, et al. Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav. 2019;3:513–25.
Hatoum AS, Morrison CL, Mitchell EC, Lam M, Benca-Bachman CE, Reineberg AE, et al. Genome-wide association study shows that executive functioning is influenced by GABAergic processes and is a neurocognitive genetic correlate of psychiatric disorders. Biol Psychiatry. 2023;93:59–70.
Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51:584–91.
Koskeridis F, Evangelou E, Said S, Boyle JJ, Elliott P, Dehghan A, et al. Pleiotropic genetic architecture and novel loci for C-reactive protein levels. Nat Commun. 2022;13:6939.
Pasman JA, Verweij KJH, Gerring Z, Stringer S, Sanchez-Roige S, Treur JL, et al. GWAS of lifetime cannabis use reveals new risk loci, genetic overlap with psychiatric traits, and a causal influence of schizophrenia. Nat Neurosci. 2018;21:1161–70.
Hill WD, Marioni RE, Maghzian O, Ritchie SJ, Hagenaars SP, McIntosh AM, et al. A combined analysis of genetically correlated traits identifies 187 loci and a role for neurogenesis and myelination in intelligence. Mol Psychiatry. 2019;24:169–81.
Graff M, Scott RA, Justice AE, Young KL, Feitosa MF, Barata L, et al. Genome-wide physical activity interactions in adiposity - A meta-analysis of 200,452 adults. PLoS Genet. 2017;13:e1006528.
Lam M, Trampush JW, Yu J, Knowles E, Davies G, Liewald DC, et al. Large-scale cognitive GWAS meta-analysis reveals tissue-specific neural expression and potential nootropic drug targets. Cell Rep. 2017;21:2597–613.
Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–21.
Lu Y, Day FR, Gustafsson S, Buchkovich ML, Na J, Bataille V, et al. New loci for body fat percentage reveal link between adiposity and cardiometabolic disease risk. Nat Commun. 2016;7:10495.
Mao K, Zhang M, Cao J, Zhao X, Gao L, Fu L, et al. Coding variants are relevant to the expression of obesity-related genes for pediatric adiposity. Obesity (Silver Spring). 2021;29:194–203.
Pearce LR, Joe R, Doche ME, Su H-W, Keogh JM, Henning E, et al. Functional characterization of obesity-associated variants involving the α and β isoforms of human SH2B1. Endocrinology. 2014;155:3219–26.
Pisanu C, Williams MJ, Ciuculete DM, Olivo G, Del Zompo M, Squassina A, et al. Evidence that genes involved in hedgehog signaling are associated with both bipolar disorder and high BMI. Transl Psychiatry. 2019;9:315.
Rask-Andersen M, Karlsson T, Ek WE, Johansson Å. Genome-wide association study of body fat distribution identifies adiposity loci and sex-specific genetic effects. Nat Commun. 2019;10:339.
Riveros-McKay F, Mistry V, Bounds R, Hendricks A, Keogh JM, Thomas H, et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet. 2019;15:e1007603.
Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48.
Volckmar A-L, Bolze F, Jarick I, Knoll N, Scherag A, Reinehr T, et al. Mutation screen in the GWAS derived obesity gene SH2B1 including functional analyses of detected variants. BMC Med Genomics. 2012;5:65.
Volckmar A-L, Song J-Y, Jarick I, Pütter C, Göbel M, Horn L, et al. Fine mapping of a GWAS-derived obesity candidate region on chromosome 16p11.2. PLoS ONE. 2015;10:e0125660.
Shadrin AA, Kaufmann T, van der Meer D, Palmer CE, Makowski C, Loughnan R, et al. Vertex-wise multivariate genome-wide association study identifies 780 unique genetic loci associated with cortical morphology. Neuroimage. 2021;244:118603.
van der Meer D, Frei O, Kaufmann T, Shadrin AA, Devor A, Smeland OB, et al. Understanding the genetic determinants of the brain with MOSTest. Nat Commun. 2020;11:3512.
van der Meer D, Kaufmann T, Shadrin AA, Makowski C, Frei O, Roelfs D, et al. The genetic architecture of human cortical folding. Sci Adv. 2021;7:eabj9446.
Sidharthan NP, Minchin RF, Butcher NJ. Cytosolic sulfotransferase 1A3 is induced by dopamine and protects neuronal cells from dopamine toxicity: role of D1 receptor-N-methyl-D-aspartate receptor coupling. J Biol Chem. 2013;288:34364–74.
Yasuda S, Liu M-Y, Suiko M, Sakakibara Y, Liu M-C. Hydroxylated serotonin and dopamine as substrates and inhibitors for human cytosolic SULT1A3. J Neurochem. 2007;103:2679–89.
Castrellon JJ, Meade J, Greenwald L, Hurst K, Samanez-Larkin GR. Dopaminergic modulation of reward discounting in healthy rats: a systematic review and meta-analysis. Psychopharmacology (Berl). 2021;238:711–23.
Wagner B, Clos M, Sommer T, Peters J. Dopaminergic modulation of human intertemporal choice: a diffusion model analysis using the D2-receptor antagonist haloperidol. J Neurosci. 2020;40:7936–48.
Chen K-W, Chang Y-J, Chen L. SH2B1 orchestrates signaling events to filopodium formation during neurite outgrowth. Commun Integr Biol. 2015;8:e1044189.
Rui L, Herrington J, Carter-Su C. SH2-B is required for nerve growth factor-induced neuronal differentiation. J Biol Chem. 1999;274:10590–4.
Shih C-H, Chen C-J, Chen L. New function of the adaptor protein SH2B1 in brain-derived neurotrophic factor-induced neurite outgrowth. PLoS ONE. 2013;8:e79619.
Zhang Y, Zhu W, Wang Y-G, Liu X-J, Jiao L, Liu X, et al. Interaction of SH2-Bbeta with RET is involved in signaling of GDNF-induced neurite outgrowth. J Cell Sci. 2006;119:1666–76.
Jiang L, Su H, Keogh JM, Chen Z, Henning E, Wilkinson P, et al. Neural deletion of Sh2b1 results in brain growth retardation and reactive aggression. FASEB J. 2018;32:1830–40.
Doche ME, Bochukova EG, Su H-W, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J Clin Invest. 2012;122:4732–6.
Duan C, Yang H, White MF, Rui L. Disruption of the SH2-B gene causes age-dependent insulin resistance and glucose intolerance. Mol Cell Biol. 2004;24:7435–43.
Ren D, Li M, Duan C, Rui L. Identification of SH2-B as a key regulator of leptin sensitivity, energy balance, and body weight in mice. Cell Metab. 2005;2:95–104.
Ren D, Zhou Y, Morris D, Li M, Li Z, Rui L. Neuronal SH2B1 is essential for controlling energy and glucose homeostasis. J Clin Invest. 2007;117:397–406.
Du X, Yan Y, Yu J, Zhu T, Huang C-C, Zhang L, et al. SH2B1 tunes hippocampal ERK signaling to influence fluid intelligence in humans and mice. Research (Wash D C). 2023;6:0269.
Gokhale A, Lee CE, Zlatic SA, Freeman AAH, Shearing N, Hartwig C, et al. Mitochondrial proteostasis requires genes encoded in a neurodevelopmental syndrome locus. J Neurosci. 2021;41:6596–616.
Coleman JRI, Bryois J, Gaspar HA, Jansen PR, Savage JE, Skene N, et al. Biological annotation of genetic loci associated with intelligence in a meta-analysis of 87,740 individuals. Mol Psychiatry. 2019;24:182–97.
Rein B, Yan Z. 16p11.2 copy number variations and neurodevelopmental disorders. Trends Neurosci. 2020;43:886–901.
Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–70.
Gill R, Chen Q, D’Angelo D, Chung WK. Eating in the absence of hunger but not loss of control behaviors are associated with 16p11.2 deletions. Obesity (Silver Spring). 2014;22:2625–31.
Hippolyte L, Maillard AM, Rodriguez-Herreros B, Pain A, Martin-Brevet S, Ferrari C, et al. The number of genomic copies at the 16p11.2 locus modulates language, verbal memory, and inhibition. Biol Psychiatry. 2016;80:129–39.
Jacquemont S, Reymond A, Zufferey F, Harewood L, Walters RG, Kutalik Z, et al. Mirror extreme BMI phenotypes associated with gene dosage at the chromosome 16p11.2 locus. Nature. 2011;478:97–102.
Maillard AM, Ruef A, Pizzagalli F, Migliavacca E, Hippolyte L, Adaszewski S, et al. The 16p11.2 locus modulates brain structures common to autism, schizophrenia and obesity. Mol Psychiatry. 2015;20:140–7.
Kusenda M, Vacic V, Malhotra D, Rodgers L, Pavon K, Meth J, et al. The influence of microdeletions and microduplications of 16p11.2 on global transcription profiles. J Child Neurol. 2015;30:1947–53.
Zufferey F, Sherr EH, Beckmann ND, Hanson E, Maillard AM, Hippolyte L, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet. 2012;49:660–8.
Adams, M.J. et al. Trans-ancestry genome-wide study of depression identifies 697 associations implicating cell types and pharmacotherapies. Cell 2025;188:640–52.e9.
Sanchez-Roige S, Barnes SA, Mallari J, Wood R, Polesskaya O, Palmer AA. A mutant allele of glycoprotein M6-B (Gpm6b) facilitates behavioral flexibility but increases delay discounting. Genes Brain Behav. 2022;21:e12800.
Cai H, Chen J, Liu S, Zhu J, Yu Y. Brain functional connectome-based prediction of individual decision impulsivity. Cortex. 2020;125:288–98.
McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–7.
Wang Q, Lv C, He Q, Xue G. Dissociable fronto-striatal functional networks predict choice impulsivity. Brain Struct Funct. 2020;225:2377–86.
Wang Q, Zhu Y, Wang Y, Chen C, He Q, Xue G. Intrinsic non-hub connectivity predicts human inter-temporal decision-making. Brain Imaging Behav. 2021;15:2005–16.
Rosch KS, Batschelett MA, Crocetti D, Mostofsky SH, Seymour KE. Sex differences in atypical fronto-subcortical structural connectivity among children with attention-deficit/hyperactivity disorder: associations with delay discounting. Behav Brain Res. 2023;452:114525.
Jentsch JD, Ashenhurst JR, Cervantes MC, Groman SM, James AS, Pennington ZT. Dissecting impulsivity and its relationships to drug addictions. Ann N Y Acad Sci. 2014;1327:1–26.
Jackson JNS, MacKillop J. Attention-Deficit/Hyperactivity disorder and monetary delay discounting: a meta-analysis of case-control studies. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:316–25.
Lee NC, de Groot RHM, Boschloo A, Dekker S, Krabbendam L, Jolles J. Age and educational track influence adolescent discounting of delayed rewards. Front Psychol. 2013;4:993.
Exum AC, Sutton CA, Bellitti JS, Yi R, Fazzino TL. Delay discounting and substance use treatment outcomes: a systematic review focused on treatment outcomes and discounting methodology. J Subst Use Addict Treat. 2023;149:209037.
Conrod PJ. Personality-targeted interventions for substance use and misuse. Curr Addict Rep. 2016;3:426–36.
Rung JM, Madden GJ. Experimental reductions of delay discounting and impulsive choice: A systematic review and meta-analysis. J Exp Psychol Gen. 2018;147:1349–81.
Vassileva J, Conrod PJ. Impulsivities and addictions: a multidimensional integrative framework informing assessment and interventions for substance use disorders. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180137.
Barrett ME, Battjes RJ. Factors associated with elevated risk of HIV among Hispanic IVDAs. NIDA Res Monogr. 1990;105:348–50.
Bidwell LC, McGeary JE, Gray JC, Palmer RHC, Knopik VS, MacKillop J. NCAM1-TTC12-ANKK1-DRD2 variants and smoking motives as intermediate phenotypes for nicotine dependence. Psychopharmacology (Berl). 2015;232:1177–86.
Brennaman LH, Maness PF. NCAM in neuropsychiatric and neurodegenerative disorders. Adv Exp Med Biol. 2010;663:299–317.
Chu C, Gao Y, Lan X, Thomas A, Li S. NCAM mimetic peptides: potential therapeutic target for neurological disorders. Neurochem Res. 2018;43:1714–22.
Conboy L, Bisaz R, Markram K, Sandi C. Role of NCAM in emotion and learning. Adv Exp Med Biol. 2010;663:271–96.
Dubertret C, Bardel C, Ramoz N, Martin P-M, Deybach J-C, Adès J, et al. A genetic schizophrenia-susceptibility region located between the ANKK1 and DRD2 genes. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:492–9.
Gelernter J, Yu Y, Weiss R, Brady K, Panhuysen C, Yang B-Z, et al. Haplotype spanning TTC12 and ANKK1, flanked by the DRD2 and NCAM1 loci, is strongly associated to nicotine dependence in two distinct American populations. Hum Mol Genet. 2006;15:3498–507.
Huang W, Payne TJ, Ma JZ, Beuten J, Dupont RT, Inohara N, et al. Significant association of ANKK1 and detection of a functional polymorphism with nicotine dependence in an African-American sample. Neuropsychopharmacology. 2009;34:319–30.
Kohl C, Riccio O, Grosse J, Zanoletti O, Fournier C, Klampfl SM, et al. The interplay of conditional NCAM-knockout and chronic unpredictable stress leads to increased aggression in mice. Stress. 2013;16:647–54.
Liu Q, Xu Y, Mao Y, Ma Y, Wang M, Han H, et al. Genetic and epigenetic analysis revealing variants in the NCAM1-TTC12-ANKK1-DRD2 cluster associated significantly with nicotine dependence in Chinese Han Smokers. Nicotine Tob Res. 2020;22:1301–9.
Mota NR, Rovaris DL, Kappel DB, Picon FA, Vitola ES, Salgado CAI, et al. NCAM1-TTC12-ANKK1-DRD2 gene cluster and the clinical and genetic heterogeneity of adults with ADHD. Am J Med Genet B Neuropsychiatr Genet. 2015;168:433–44.
Yang B-Z, Kranzler HR, Zhao H, Gruen JR, Luo X, Gelernter J. Association of haplotypic variants in DRD2, ANKK1, TTC12 and NCAM1 to alcohol dependence in independent case control and family samples. Hum Mol Genet. 2007;16:2844–53.
Mitchell SH. Assessing delay discounting in mice. Curr Protoc Neurosci. 2014;66:Unit 8.30.
Mitchell SH. Linking delay discounting and substance use disorders: genotypes and phenotypes. Perspect Behav Sci. 2019;42:419–32.
Gustavson DE, Franz CE, Kremen WS, Carver CS, Corley RP, Hewitt JK, et al. Common genetic influences on impulsivity facets are related to goal management, psychopathology, and personality. J Res Pers. 2019;79:161–75.
Gustavson DE, Friedman NP, Fontanillas P, Elson SL, Palmer AA, Sanchez-Roige S. The latent genetic structure of impulsivity and its relation to internalizing psychopathology. Psychol Sci. 2020;31:1025–35.
Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, de Wit H, MacKillop J, et al. Genome-wide association studies of impulsive personality traits (BIS-11 and UPPS-P) and drug experimentation in up to 22,861 adult research participants identify loci in the CACNA1I and CADM2 genes. J Neurosci. 2019;39:2562–72.
Sanchez-Roige S, Jennings MV, Thorpe HHA, Mallari JE, van der Werf LC, Bianchi SB, et al. CADM2 is implicated in impulsive personality and numerous other traits by genome- and phenome-wide association studies in humans and mice. Transl Psychiatry. 2023;13:167.
Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80:28–44.
Sanchez-Roige S, Palmer AA. Emerging phenotyping strategies will advance our understanding of psychiatric genetics. Nat Neurosci. 2020;23:475–80.
Aschard H, Vilhjálmsson BJ, Joshi AD, Price AL, Kraft P. Adjusting for heritable covariates can bias effect estimates in genome-wide association studies. Am J Hum Genet. 2015;96:329–39.
Sanchez-Roige S, Fontanillas P, Elson SL, Gray JC, de Wit H, Davis LK, et al. Genome-wide association study of alcohol use disorder identification test (AUDIT) scores in 20 328 research participants of European ancestry. Addict Biol. 2019;24:121–31.
Border R, Athanasiadis G, Buil A, Schork AJ, Cai N, Young AI, et al. Cross-trait assortative mating is widespread and inflates genetic correlation estimates. Science. 2022;378:754–61.
Deng WQ, Belisario K, Munafò MR, MacKillop J. Longitudinal characterization of impulsivity phenotypes boosts signal for genomic correlates and heritability. Mol Psychiatry. 2025;30:608–18.
Sollis E, Mosaku A, Abid A, Buniello A, Cerezo M, Gil L, et al. The NHGRI-EBI GWAS Catalog: knowledgebase and deposition resource. Nucleic Acids Res. 2023;51:D977–85.
Watanabe K, Umićević Mirkov M, de Leeuw CA, van den Heuvel MP, Posthuma D. Genetic mapping of cell type specificity for complex traits. Nat Commun. 2019;10:3222.
Altshuler DM, Gibbs RA, Peltonen L, Altshuler DM, Gibbs RA, Peltonen L, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58.
Rosenthal SB, Willsey HR, Xu Y, Mei Y, Dea J, Wang S, et al. A convergent molecular network underlying autism and congenital heart disease. Cell Syst. 2021;12:1094–107.e6.
Rosenthal SB, Wright SN, Liu S, Churas C, Chilin-Fuentes D, Chen C-H, et al. Mapping the common gene networks that underlie related diseases. Nat Protoc. 2023;18:1745–59.
Wright SN, Leger BS, Rosenthal SB, Liu SN, Jia T, Chitre AS, et al. Genome-wide association studies of human and rat BMI converge on synapse, epigenome, and hormone signaling networks. Cell Rep. 2023;42:112873.
Vanunu O, Magger O, Ruppin E, Shlomi T, Sharan R. Associating genes and protein complexes with disease via network propagation. PLoS Comput Biol. 2010;6:e1000641.
Guney E, Menche J, Vidal M, Barábasi A-L. Network-based in silico drug efficacy screening. Nat Commun. 2016;7:10331.
Singhal A, Cao S, Churas C, Pratt D, Fortunato S, Zheng F, et al. Multiscale community detection in Cytoscape. PLoS Comput Biol. 2020;16:e1008239.
Kuleshov MV, Jones MR, Rouillard AD, Fernandez NF, Duan Q, Wang Z, et al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016;44:W90–7.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504.
Roden DM, Pulley JM, Basford MA, Bernard GR, Clayton EW, Balser JR, et al. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–9.
Ge T, Chen C-Y, Ni Y, Feng Y-CA, Smoller JW. Polygenic prediction via Bayesian regression and continuous shrinkage priors. Nat Commun. 2019;10:1776.
Denny JC, Bastarache L, Roden DM. Phenome-wide association studies as a tool to advance precision medicine. Annu Rev Genomics Hum Genet. 2016;17:353–73.
Bell N, Uffelmann E, van Walree E, de Leeuw C, Posthuma D. Using genome-wide association results to identify drug repurposing candidates. medRxiv. 2022. [Preprint] 2022. https://doi.org/10.1101/2022.09.06.22279660.
Acknowledgements
J.J.M., L.V.R., A.A.P., and S.S.R. are funded through the National Institute on Drug Abuse (NIDA DP1DA054394 and P50DA037844). R.B.C., S.R.P., M.V.J., A.A.P., and S.S.R. are funded through the Tobacco-Related Disease Research Program (T32IR5226 and 28IR-0070). H.H.A.T. is supported by a Canadian Institute of Health Research (CIHR) postdoctoral fellowship (#491556). R.B.C is also funded by the Interdisciplinary Research Fellowship in NeuroAIDS (5R25MH081482-17). T.T.M. is supported by funds from the National Institute of Mental Health (K08MH135343). LKD was supported by grants from the National Institutes of Health including R01MH118223. The PheWAS analysis made use of the VUMC Synthetic Derivative (SD), which was supported by the National Center for Research Resources, Grant UL1 RR024975-01, and is now at the National Center for Advancing Translational Sciences, Grant 2 UL1 TR000445-06. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The dataset(s) used for the PheWAS/LabWAS analyses were obtained from Vanderbilt University Medical Center’s BioVU which is supported by numerous sources: institutional funding, private agencies, and federal grants. These include the NIH funded Shared Instrumentation Grant S10RR025141; and CTSA grants UL1TR002243, UL1TR000445, and UL1RR024975. Genomic data are also supported by investigator-led projects that include U01HG004798, R01NS032830, RC2GM092618, P50GM115305, U01HG006378, U19HL065962, R01HD074711; and additional funding sources listed at https://victr.vumc.org/biovu-funding/.
Ethics declarations
Competing interests
P.F., the 23andMe Research Team, and S.L.E. were employed by 23andMe, Inc. P.F. and S.L.E. hold stock or stock options in 23andMe, Inc. The remaining authors have nothing to disclose.
Ethics statement
All methods were performed in accordance with the relevant guidelines and regulations. Participants provided informed consent and volunteered to participate in research online under a protocol approved by the external AAHRPP-accredited Institutional Review Board (IRB), Ethical & Independent (E&I) Review Services.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thorpe, H.H.A., Cupertino, R.B., Pakala, S.R. et al. Genome-wide association study of delay discounting identifies 11 loci and reveals transdiagnostic associations across mental and physical health. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03356-8
Received:
Revised:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41380-025-03356-8