Findings from a study co-led by UTSW reveal a possible mechanism behind a malignancy that has risen rapidly over the past few decades.
A study co-led by researchers at UT Southwestern Medical Center suggests that long-term inflammation can make the colon more rigid, a change that may promote the onset and growth of colorectal cancer diagnosed at younger ages. Published in Advanced Science, the findings point to new opportunities for preventing and treating this particularly aggressive form of colorectal cancer (CRC).
“We consider this study a significant advancement toward identifying those at risk of early-onset CRC and finding new ways to treat them,” said Emina Huang, M.D., M.B.A., Professor of Surgery in the Division of Colon and Rectal Surgery and Executive Vice Chair of Research for Surgery at UT Southwestern. She is also Professor of Biomedical Engineering and in the Harold C. Simmons Comprehensive Cancer Center.
UT Southwestern partnered with researchers from The University of Texas at Dallas on the study.

“This is the first study to highlight the key role of biomechanical forces in the pathogenesis of early-onset CRC,” said Jacopo Ferruzzi, Ph.D., Assistant Professor of Bioengineering at UT Dallas and Biomedical Engineering at UT Southwestern. “Our observations are consistent across multiple length scales and link connective tissue stiffening to altered biochemical signaling in cancer cells.”
Early-onset CRC is rising, causes unclear
Colorectal cancers that develop without inherited genetic syndromes and typically appear after age 50 are referred to as average-onset or sporadic CRCs. Over the past 30 years, both the number of new cases and deaths from these cancers has steadily declined. In contrast, colorectal cancers diagnosed before age 50, known as early-onset CRCs, have increased sharply over the same time frame. Since 2020, early-onset cases have made up roughly 12 percent of all colorectal cancer diagnoses in the United States.
The cause of this rise remains unclear. Much of the existing research has examined factors such as diet, obesity, and environmental exposures that may contribute to chronic inflammation in the gut. What has been less well understood is how ongoing intestinal inflammation could specifically trigger the development of colorectal cancer at younger ages.
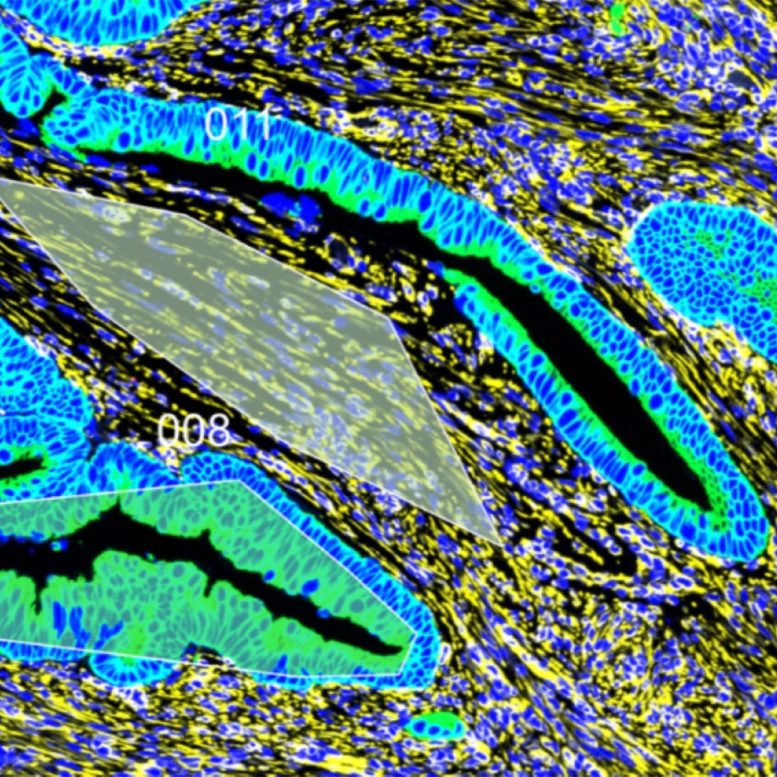
Inflammation stiffens tissue before cancer
Dr. Huang explained that chronic inflammation can cause scarring, gradually increasing the stiffness of tissues over time. Such stiffness is known to drive development and progression in some other cancer types, such as breast and pancreatic cancers. She and her colleagues wondered whether a similar phenomenon might spur early-onset CRC.
To answer this question, researchers worked with intestinal tissue from patients who underwent surgery to remove their cancerous tumors at William P. Clements University Hospital and Parkland Health: 19 samples from patients with average-onset CRC and 14 from patients with early-onset CRC. Each sample included not only malignant tumors but also their noncancerous margins. Tests showed that both the tumors and the noncancerous tissue were significantly stiffer in samples from patients with early-onset CRC compared with those from patients with average-onset CRC. These findings suggest that an increase in stiffness may have preceded early-onset CRC development.

Searching for a reason for this increased rigidity, researchers examined the collagen in both sample types, a protein that increases in abundance and changes conformation with scarring. They found that collagen in the early-onset samples was denser, longer, more mature, and more aligned than those in the average-onset samples. Those factors underscore the role of scarring in early-onset CRC tissue.
When scientists compared gene activity in the two sample types, they saw a significant increase in the expression of genes associated with collagen metabolism, blood vessel formation, and inflammation in the early-onset CRC tissues, further reinforcing that scarring from chronic inflammation is responsible for tissue stiffness. Importantly, they also noticed an uptick in a molecular pathway responsible for mechanotransduction, a process in which cells convert mechanical forces into biochemical signals. This suggests that cells in the early-onset CRC samples might change their behavior based on the stiffness of their environment.
Stiffness drives cancer behavior and growth
Not surprisingly, when the researchers grew CRC cell lines on substrates with various levels of rigidity, they found that the cells multiplied quicker on stiffer substrates and increased rigidity. Similarly, three-dimensional organoid models made from CRC cells grew bigger faster on stiffer substrates.
Together, Dr. Huang said, these findings suggest that a more rigid environment might cause CRC to initiate and grow in those who develop early-onset CRC. They also reinforce the idea that disrupting mechanotransduction molecular pathways in these cells could halt or reverse CRC initiation and growth, a strategy currently being explored for some other cancers. Developing diagnostic tests to assess intestinal stiffness could help identify those at risk of early-onset CRC, Dr. Huang added, much like colonoscopies have done for average-onset CRC.
Reference: “Biomechanical Phenotyping Reveals Unique Mechanobiological Signatures of Early-Onset Colorectal Cancer” by Nicole C. Huning, Munir H. Buhaya, Victor V. Nguyen, Afeefah Khazi-Syed, Haider A. Ali, Adil Khan, Angela Fan, Robert C. Fisher, Zhikai Chi, Indu Raman, Guangchun Chen, Chengsong Zhu, Mengxi Yu, Andrew R. Jamieson, Sara Roccabianca, Victor D. Varner, Cheryl M. Lewis, Emina H. Huang and Jacopo Ferruzzi, 1 December 2025, Advanced Science.
DOI: 10.1002/advs.202514693
This study was funded by the National Institutes of Health (R01 CA234307 and U01 CA214300), The University of Texas at Dallas Office of Research and Innovation through the CoBRA program, the Burroughs-Wellcome Trust, the American Society of Colon and Rectal Surgeons Resident Research Initiation Grant, The University of Texas at Dallas Bioengineering Research Award, the UT Southwestern Whole Brain Microscopy Facility, an Axioscan 7 Award, the Catherine and James McCormick Charitable Foundation supporting research in early-onset colorectal cancer, and a National Cancer Institute (NCI) Cancer Center Support Grant (P30 CA142543).
Never miss a breakthrough: Join the SciTechDaily newsletter.
Follow us on Google and Google News.